Vedeli ste, že ...?
Z hľadiska účinku sú generické lieky rovnako účinné ako originálne lieky.
Vďaka generickým liekom je možné odliečiť dvojnásobné množstvo pacientov bez negatívneho dopadu na náklady vynaložené na lieky zo zdrojov verejného zdravotníctva.
Generické lieky musia spĺňať rovnaké prísne štandardy ako všetky registrované lieky, vrátane originálnych liekov, a garantom ich kvality, bezpečnosti a účinnosti sú štátne inštitúcie.
Používaním generík je možné viac finančných zdrojov vrátiť späť do zdravotného systému a následne ich investovať do prevencie, diagnostiky rôznych ochorení ako aj ich liečby inovatívnymi liekmi.
Generické lieky môžu mať inú farbu, tvar alebo veľkosť v porovnaní s originálom, ktoré však nemajú vplyv na ich účinnosť, bezpečnosť, ani kvalitu - sú garantované liekovými agentúrami.
Celkové klinické skúsenosti s biosimilárnymi liekmi, schválenými v EÚ presahujú 2 miliardy pacientodní.
Za posledných 10 rokov sa kumulatívny počet pacientodní s biosimilárnou liečbou schválenou v EÚ sa zdvojnásobil každého 1,5 roka.
Lieky s pridanou hodnotou vznikajú inováciou generických liekov a zlepšujú adherenciu pacientov k liečbe a kvalitu ich života.
Generiká predstavujú takmer 70 % predpísaných liekov v Európe, pričom však predstavujú menej ako 30 % výdavkov na lieky.
Pre väčšinu chronických ochorení,- ako je cukrovka, vysoký cholesterol, vysoký krvný tlak -, generické lieky sú liekom prvej voľby. V rámci EÚ 80 % výdavkov v zdravotníctve sa spája s chronickými ochoreniami.
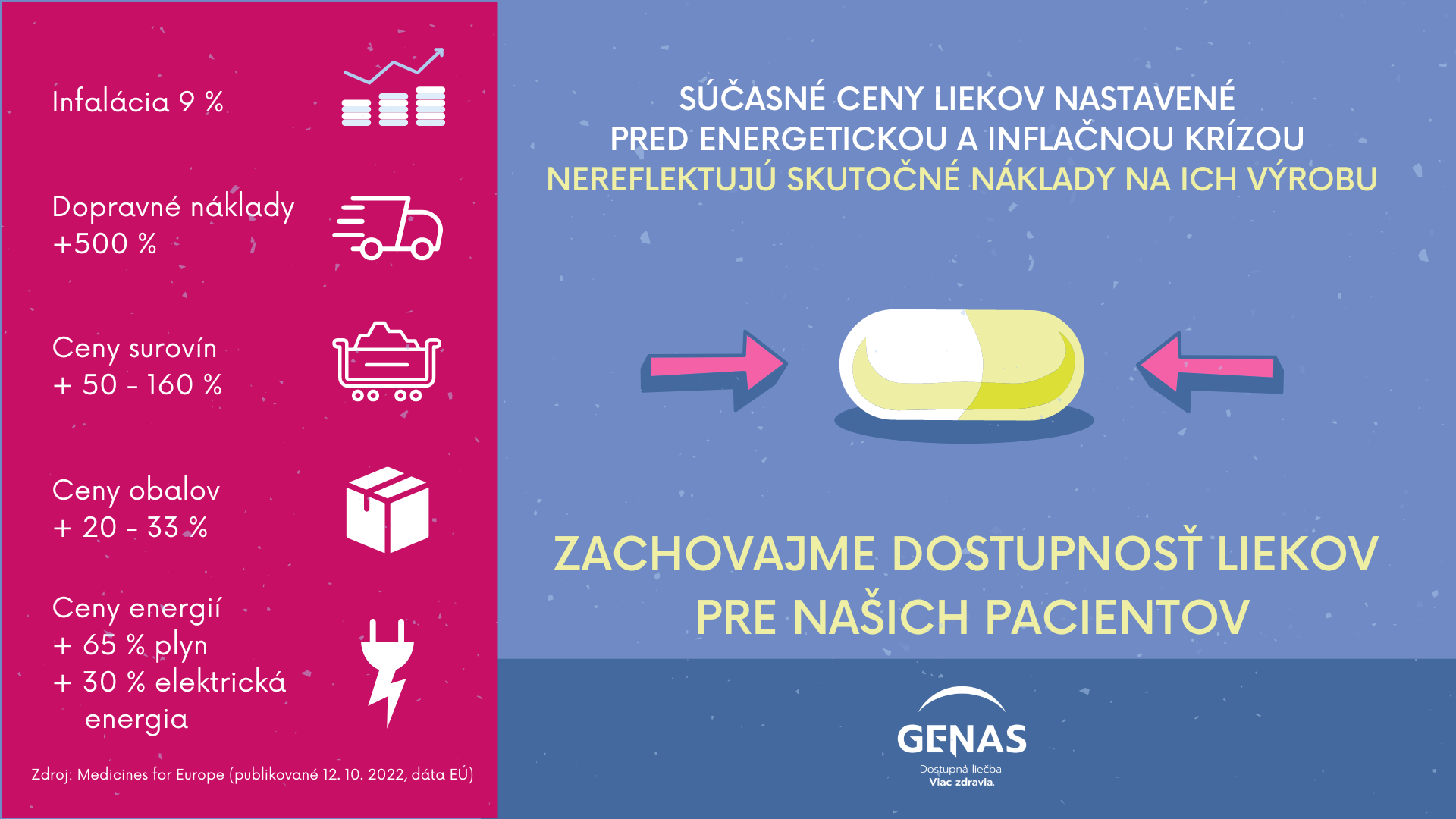
Infografiky